 Medtech strategy and business development | MDxp
Medtech strategy and business development | MDxpMDxp helps you to obtain the "Assurance Prospection Accompagnement" financed by BPI France, Team France Export and the French government.



Starting out in export? BPI France, Team France Export and the French government provide financial support to finance your first prospecting actions up to a guaranteed budget of 40,000 euros.
More about the program
We work with Health Tech innovators (medical devices, digital health...), whether they are project leaders or international companies, in order to support them in the success of their projects.
This is what we do at MDxp.
Commercial brochure.

- growth and development strategy
- valuation of technologies
- assistance with business plans
- fund raising request
- recruitment

- sales strategy
- geo-targeted marketing strategy
- market access strategy :
reimbursement, clinical studies, scientific board, regulatory...

- operating management
- strategies deployment
- searching for distributors
- searching for the right partners

MDxp, along with its sister company MD101, has put together a list of training modules for medical device professionals looking to expand their business worldwide.
This is what we do at MDxp.

• 21 years experience • sales manager • business development specialist • international marketing expert
LinkedInMathieu brings over 18 years of experience in international business development and international commercialization of innovative medical devices. With extensive experience with both startups and large corporations, his ambitions are to consistently bring value to his clients while helping to bring innovative devices to market for the benefit of patients.

• 13 years of experience • manager • international sales specialist • Asia expert
LinkedInRomain brings over 10 years of international sales experience, mainly focused on Asian markets (Japan, South Korea, China, Taiwan, Singapore), India and Australia. For the past 7 years, he has focused on managing international distributors as well as managing and implementing complex technical projects. With a deep knowledge of the medical imaging software field, he is now actively working hand in hand with the R&D teams of our customers, industrial partners, local distributors and end users.

• 18 years of experience • web developer • e-commerce specialist • PHP/JS/SQL/symphony expert
LinkedInWeb developer for more than 15 years now, Jerome has collaborated on more than a hundred web projects, specializing in helping companies develop their online business.
Today, he puts his skills entirely at the disposal of MDxp, MD101 and EMT.
Because an international development strategy cannot be thought of without a transversal approach, MDxp relies on MD101 to meet all your needs.
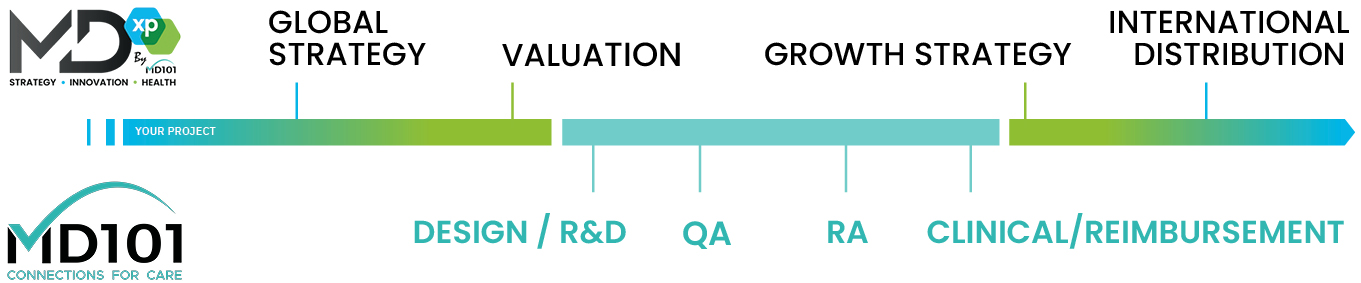

MD101 has worked with over 800 medical device and digital health manufacturers (all device types, all classes) over the past 10 years. From concept to market, MD101 is a trusted partner to its customers with over 100 experts worldwide and 30 partner companies providing complementary services.
MD101's services evolve around four pillars:
1. R&D,
2. Quality Assurance & Regulatory Affairs,
3. Clinical Strategy,
4. Reimbursement Strategy.
MD101's unique market positioning makes it a reliable and robust service provider for all medtech / ehealth companies, from startups to multinational organizations.
MDxp is now working closely with MD101 to help manufacturers bring their innovations to market with the help of distributors or industrial partners. As specialists in medical technology and digital health, we thrive on working on sales and marketing strategies, working on business plans / models, developing the right international sales strategy, developing and managing distribution networks...The complementarity is logical and of high added-value for our clients.
All MD101 services

The alliance of seven expertise: a UNIQUE approach for your project.
Seven recognized boutique consulting firms in healthtech to accompany you from the birth to the completion of your project :
• idea: Anticipate and protect your innovation.
• implementation: making your innovation effective.
• compliance: comply with market requirements.
• commercialization: developing and preventing risks.
Take advantage of the experience of the Alliance members by participating in the Medtech Experts Meetings, events that have become a must-attend since 2015.
Roundtables followed by 20-minute face-to-face meetings with our experts !! Registration for #pointsEMT is free.
LinkedIn
Here are some examples of medical devices that we strive to bring to different markets with the help of qualified distributors.
You are a manufacturer and want to develop your international presence? Contact us!

by Hirondelle medical

 Hirondelle Medical has developed a unique platform bringing a new and simplified technique to osteoarticular interventional procedures.
Hirondelle Medical has developed a unique platform bringing a new and simplified technique to osteoarticular interventional procedures.
by Smade

 Self-powered devices that collect and deliver invaluable data to the cloud. Smade : Where digital codes enhances physical tools.
Self-powered devices that collect and deliver invaluable data to the cloud. Smade : Where digital codes enhances physical tools.
by Revinax

 Immersive tutorials for healthcare providers and specialized nurses - Learn, review, and maintain your skills.
Immersive tutorials for healthcare providers and specialized nurses - Learn, review, and maintain your skills.They joined us in the adventure and bring a strong added value to our clients and an excellent service.


ICOSA is an intellectual property firm dedicated to the Health sector.
Learn more about ICOSA


The expertise of seven major players in Medtech: from the idea to the success of your project.
We help innovation manufacturers succeed by intervening across the entire value chain and at all stages of your innovation's development.
Learn more about the .EMT Alliance


Audit & RiskSolutions is an insurance broker dedicated to Life Sciences industry including biotechnologies companies, medical device manufacturers, laboratories, contract and clinical research facilities, pharmaceutical companies.
They provide their clients with a wide range of insurance products such as property insurance, professional and product liability insurance, D&O, clinical trial insurance.
Learn more about Audit & RiskSolutions


EUROBIOMED is the lifesciences cluster of the south of France, assisting from fundamental research projects to bringing to market, for the benefit of patients.
Learn more about EUROBIOMED


MedTech Momentum is a full-service marketing firm, in business to craft and execute results-oriented online marketing strategies in the medical space. It has a partner for a number of years of MD101 & MDxp.
Learn more about Medtech Momentum


MEAMED, our partner in Middle-East, Africa and South Asia.
Since 2008, we supported established, emerging and start up medical devices manufacturers, to establish and/or develop a successful sales\distributor networks within the region.
Learn more about MEAMED


SPEBA INNOVATIONS, Bringing medical technologies closer to patients.
We at Speba are dedicated to introducing cutting-edge and innovative medical technologies, interfaced with e-solutions, helping healthcare professionals to better treat and care for their patients, and giving patients easy access to such advanced treatment modalities thus enabling them to take control of and manage their health themselves, their improved quality of life and leading normal or close to normal lives
Learn more about SPEBA INNOVATIONS


La French Tech is the French startup movement. A unique ecosystem that uses startups (therefore), but also investors, decision-makers and community builders. Our mission: to make France one of the most attractive countries in the world for startups who want to get started, conquer international markets and build a meaningful future. In video, it's better!
Learn more about La French Tech


Located in Strasbourg, PROTOMED is a contract service R&D company specialized in medical device design, development and testing. PROTOMED has spun-offs its device testing laboratory ProtomedLabs and has participated to many developments in minimally invasive surgery, single use devices, vascular and cardiac implants.
Its fablab includes prototyping machines (3D printers; CNC machines), test benches, control equipments and state of the art CAD software.
Learn more about PROTOMED


We support your innovation efforts by helping you obtain the financing you need to bring your company to the next level. We are specialized in EU funding schemes such as Horizon 2020, Eureka-Eurostars, ERA-NETs , Life and Interreg. We provide a range of services, from defining your funding strategy to writing your funding proposal.
Learn more about Futuro Perfecto


OCO Global is a technology-enabled advisory firm focused on trade, investment and economic development.
We work with our clients to ensure that their international business thrives, by delivering innovative and sustainable outcomes and connecting them to opportunities across the world that create prosperity, employment and economic growth.
Learn more about OCO Global

 The OSCI is the federation of private companies dedicated to the international development of companies.
The OSCI is the federation of private companies dedicated to the international development of companies.
Whatever their size, their expertise and their geographical presence, our members work alongside managers and their teams, players in international trade and across the entire value chain of the internationalization of French companies.
Their specialized services by trade, geographical sector or sector of activity make it possible to support strategic thinking and ensure the international deployment of French exporting companies.
OSCIs share common expertise in the field and a culture of results that make them natural partners for companies.
Learn more about l'OSCI
Feel free to reach out to us & we'll be happy to discuss further.